No Data
US OptionsDetailed Quotes
TFX250516P190000
- 0.00
- 0.000.00%
15min DelayTrading Apr 30 09:30 ET
0.00High0.00Low
0.00Open0.00Pre Close0 Volume0 Open Interest190.00Strike Price0.00Turnover101.55%IV-40.42%PremiumMay 16, 2025Expiry Date54.69Intrinsic Value100Multiplier16DDays to Expiry0.00Extrinsic Value100Contract SizeAmericanOptions Type-0.9329Delta0.0049Gamma2.46Leverage Ratio-0.0997Theta-0.0497Rho-2.29Eff Leverage0.0367Vega
Intraday
- 5D
- Daily
No Data
News
Teleflex to Present at the BofA Securities 2025 Health Care Conference
The FDA Has Granted 510(k) Clearance Of An Expansion To The Indications For Use Of Teleflex's QuikClot Control+ Hemostatic Device To Include All Grades Of Internal And External Bleeding
Teleflex Receives FDA 510(k) Clearance for Expanded Indications of the QuikClot Control+ Hemostatic Device
Express News | Teleflex Receives FDA 510(K) Clearance for Expanded Indications of the Quikclot Control+™ Hemostatic Device
Earnings Preview: Teleflex to Report Financial Results Pre-market on May 01
Teleflex Announces Clinical and Real-World Evidence Studies and Physician Educational Opportunities to Be Highlighted at the 2025 American Urological Association (AUA) Annual Meeting
Comments
$Teleflex (TFX.US)$
FDA Approval Unlocks $150M Market: Teleflex's QuikClot Now Cleared for All Bleeding Grades
FDA Approval Unlocks $150M Market: Teleflex's QuikClot Now Cleared for All Bleeding Grades

The market started mixed and flat, and ended low. Nvidia's earnings were not enough to turn around a tech crunch that kept squeezing high-priced Magnificent Seven stocks. President Trump said Thursday morning that 25% tariffs on Canada and Mexico, and 10% more tariffs on China were coming next week.
Just past 4 pm ET the $S&P 500 Index (.SPX.US)$ fell 1.59%, the $Dow Jones Industrial Average (.DJI.US)$ fell 0.45%, and the $Nasdaq Composite Index (.IXIC.US)$ fell 2.78...
Just past 4 pm ET the $S&P 500 Index (.SPX.US)$ fell 1.59%, the $Dow Jones Industrial Average (.DJI.US)$ fell 0.45%, and the $Nasdaq Composite Index (.IXIC.US)$ fell 2.78...

36
3
19
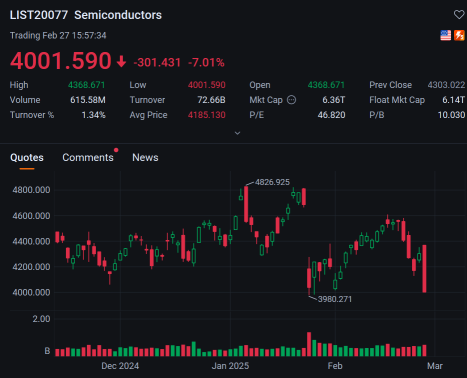
Happy Thursday investors, unless you are a Nvidia fan. It's February 27th, and the market is trying to reach higher even with its semiconductor darling falling after its report, how could that be possible?
Check in to the live stock news to find out!
$Warner Bros Discovery (WBD.US)$ climbed 8%, the highest on the S&P after the firm posted earnings that impressed investors: boasting studio revenue growth of 16%, and forecasting 150 m...
Check in to the live stock news to find out!
$Warner Bros Discovery (WBD.US)$ climbed 8%, the highest on the S&P after the firm posted earnings that impressed investors: boasting studio revenue growth of 16%, and forecasting 150 m...

14
6
8
$Teleflex (TFX.US)$
Teleflex's Bold Restructuring: Will Separating Into Two Companies Unlock Greater Shareholder Value?
Teleflex's Bold Restructuring: Will Separating Into Two Companies Unlock Greater Shareholder Value?

$Teleflex (TFX.US)$
Teleflex Makes €760M Strategic Move: How This Vascular Acquisition Boosts Earnings Potential
Teleflex Makes €760M Strategic Move: How This Vascular Acquisition Boosts Earnings Potential

1
Read more


